
C. Ola Landgren, MD, PhD, discusses the role of minimal residual disease negativity and the emergence of CAR T-cell therapy in multiple myeloma.

C. Ola Landgren, MD, PhD, discusses the role of minimal residual disease negativity and the emergence of CAR T-cell therapy in multiple myeloma.

The trial results indicate that EBV-specific adoptive T cell therapy is well tolerated and further back this approach in efficacy trials.

Chimeric antigen receptor T cells targeting the tyrosine kinase receptor ROR1 can be transferred into patients safely and the cells expand in vivo.

The use of circulating tumor-cell counts demonstrated strong value for selecting endocrine therapy versus chemotherapy for patients with estrogen receptor–positive, HER2-negative metastatic breast cancer.
A median 19-month follow-up of the JULIET trial—a single-arm, open-label, multicenter, global, pivotal phase 2 trial of the chimeric antigen receptor-T cell therapy tisagenlecleucel directed against CD19-expressing B cells—has found a 40% complete response and a manageable safety profile in adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL).

In contrast to the wide array of available therapies in the metastatic setting of renal cell carcinoma, in patients with localized disease, the results of trials investigating adjuvant systemic therapy after nephrectomy have so far been underwhelming.
A multitude of BCMA-targeted CAR T-cell therapies are currently in development, each demonstrating different efficacy and safety profiles and each with different constructs.
The therapy attempts to restore fetal hemoglobin production.

The FDA has accepted a Biologics License Application as well as granted Priority Review for the gene therapy that treats spinal muscular atrophy (SMA) Type 1.

The anti-BCMA CAR T cell therapy bb21217 demonstrated an objective response rate of 83.3%, with a very good partial response or better rate of 75% in patients with heavily pretreated relapsed/refractory multiple myeloma.

Two-year maintenance therapy with ixazomib led to a 39% improvement in progression-free survival compared with placebo in patients with newly diagnosed multiple myeloma who achieved a partial response to induction treatment with a proteasome inhibitor and/or an immunomodulatory agent following autologous stem cell transplant.

Health resource utilization data gathered from the TRANSCEND-NHL trial have found that longer stays in the intensive care unit have a significant impact on the cost of care due to cytokine release syndrome (CRS) following treatment with chimeric antigen receptor (CAR) T cells.

Checkpoint inhibition showed promise for augmenting or extending response to chimeric antigen receptor T-cell therapy in some patients with relapsed B-cell acute lymphoblastic leukemia.

Receiving a stem cell transplant for the first time following CD19 CAR T-cell therapy induced a reduction in the risk for acute lymphoblastic leukemia (ALL) recurrence, according to a retrospective analysis of the phase I/II PLAT-02 study.

Concurrent treatment with ibrutinib and the CD19-targeted chimeric antigen receptor T-cell therapy JCAR014 was well tolerated and led to an overall response rate of 83% in patients with relapsed or refractory chronic lymphocytic leukemia.

Treatment with the CD19-targeted CAR T-cell therapy tisagenlecleucel demonstrated sustained rates of relapse-free survival and overall survival at 24 and 18 months for pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia.

A multicenter retrospective study that evaluated the efficacy and safety of chimeric antigen receptor (CAR) T-cell treatment, axicabtagene ciloleucel (axi-cel; Yescarta), in a real-world setting found a similar response as well as toxicity compared with the ZUMA-1 clinical trial.

Robert J. Kreitman, MD, discusses key attributes of moxetumomab pasudotox and its potential impact in hairy cell leukemia.

Although CAR T-cell therapies have proved successful in certain hematologic malignancies, efforts to employ similar strategies in solid tumors have been challenging. Investigators are working on different forms of adoptive cell therapy in solid tumors and early signs are promising.
The combination of nivolumab (Opdivo) and ipilimumab (Yervoy) did not improve overall survival versus placebo as a maintenance therapy for patients with extensive-stage small cell lung cancer without disease progression following frontline platinum-based chemotherapy.
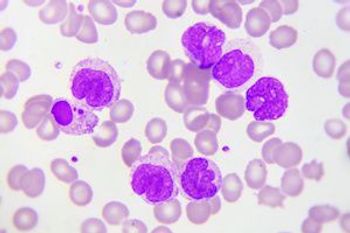
CD7 gene-edited chimeric antigen receptor (CAR) T cells target CD7-positive acute myeloid leukemia (AML) cells while sparing myeloid and erythroid cells and minimizing toxicity, according to the results of a recent study.

Stephanie Jackson, MSN, RN speaks with Cancer Network about the role oncology nurses play in managing patients with hematologic malignancies who are undergoing CAR T-cell therapy.

The FDA has granted brentuximab vedotin a breakthrough therapy designation for use in combination with chemotherapy for the frontline treatment of patients with CD30-expressing peripheral T-cell lymphoma.
The investigators hope to gain FDA approval on the treatment so they can treat more patients.

Robert Dean, MD, discusses the promise for new therapies in mantle cell lymphoma.

This fall, The American Journal of Managed Care® convened a panel of experts on migraine to discuss calcitonin gene-related peptide (CGRP) inhibitors, an emerging therapy for the condition, which affects 39 million people in the United States.

Therapies designed to treat neurologic conditions have made up 25% of the submissions to the FDA for a Regenerative Medicine Advanced Therapy designation.

There is limited data on when and whether oncologists should change systemic therapy for patients with non–small cell lung cancer, but some studies provide useful guidance.


In light of recent advancements, the current paradigm for choosing first-line therapy for patients with metastatic non–small cell lung cancer who do not harbor an actionable driver oncogene depends upon PD-L1 expression level and histology.