
An international research team has shown that optogenetic therapy has helped to partially regain visual function in a patient with retinitis pigmentosa. This is a milestone towards a gene therapy that could restore vision.


An international research team has shown that optogenetic therapy has helped to partially regain visual function in a patient with retinitis pigmentosa. This is a milestone towards a gene therapy that could restore vision.

Maria A. Croyle, PhD, discusses the manufacturing challenges facing the gene therapy space and her inspiration for developing a novel preservation method for viral vector-based therapies.

Locanabio CEO James Burns, PhD, shares his outlook on the quickly changing landscape of gene therapy research.

Although the XIRIUS study did not meet its primary end point, positive trends were observed across several clinically relevant prespecified secondary end points.

Preliminary results have shown that AGTC-401 and AGTC-402 seem safe and well tolerated in patients with ACHM.

The CEO of Locanabio shares details of 2 presentations the company is making this week at the 24th Annual Meeting of the American Society of Gene & Cell Therapy.

A podcast interview with study author Paul Yang, MD, PhD, on the current research and future implementation of the agent.

Investigators used an adeno-associated viral vector to deliver a normal functioning copy of the RPGR gene via subretinal injection.

Lineage Cell Therapeutics CEO Brian Culley shares a clinical trial update on their leading cell therapy candidate and discusses the important role of the FDA as more players enter the cell and gene therapy space.

The single-injection investigative gene therapy may help with providing continuous expression of aflibercept.

The AAV8 vector–based gene therapy was associated with improvements in 2 measures of visual function.

A non-viral gene therapy sustained drug-delivery product that delivers anti-VEGF to the eye may replace the need for repeated intravitreal anti-VEGF injections and improve vision in patients with wet AMD.

Optical coherence tomography characteristics may be predictive of the efficacy of intravitreal injection of allogeneic human retinal progenitor cells, according to research presented at ARVO 2021.

Approach can unravel causes in MYOC and TBK1 glaucoma.

A patient enrolled in the phase 2 INFINITY trial exploring ADVM-022 for diabetic macular edema experienced a suspected unexpected serious adverse reaction, which has led to an unmasking of the study.

The CEO of Lineage Cell Therapeutics discusses their unique approach to addressing degenerative vision loss in dry age-related macular degeneration.

The gene therapy SAR439483 demonstrated promising early signs of activity in patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D.

Studies are uncovering a range of potential treatment options for disorders.
Physician offers patient counseling pearls for selected retinal diseases.

AAV2 TrkB-2A-mBDNF resulted in early signs of efficacy in preclinical studies of glaucoma and humanized tauopathy, which could be translatable to other neurodegenerative polygenic disorders.

The AAV-mediate gene therapy BS01 allowed 4 patients with retinitis pigmentosa who had complete or near-complete blindness to perceive light and motion.
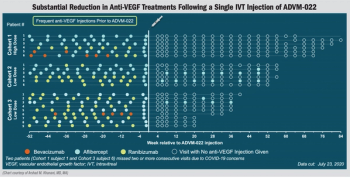
Investigators observe dramatic decrease in treatment burden seen in OPTIC study.

No approved treatment for leber hereditary optic neuropathy is currently available in the United States.
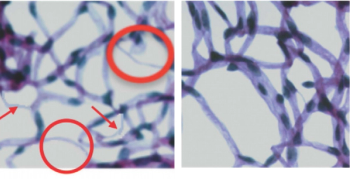
Study examining the role of IL-17A in patients with diabetes.
Investigators focus on biophysical method to study protein-protein interactions .