
Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending March 4, 2022.

David Taylor, PhD, an assistant professor in the department of molecular biosciences at UT Austin, discussed the Cas9 research conducted between 2 labs at UT Austin.

The decision is the latest action in a line of patent interferences over the revolutionary gene-editing technology.

The SARM1 gene is a key driver in the damage that ultimately leads to impaired vision.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
The professor of ophthalmology from Radboud University, The Netherlands, discussed ongoing gene therapy trials in Stargardt disease.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
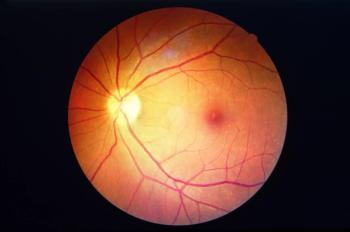
The therapy has been well-tolerated in the 15 patients treated so far.
Researchers will use the model to study how lack of pigmentation affects RPE physiology and function.

The ophthalmologist from John A. Moran Eye Center discussed the close association between disease progression and uncovered risk alleles.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Investigators did not find any clinical signs of rejection of the mismatched donor cells.
The associate professor from UC San Diego discussed the curative potential of gene therapy in cystinosis.
The associate professor from Tufts University School of Medicine discussed the latest updates on the FOCUS trial.

Review top news and interview highlights from the week ending February 11, 2022.

Clinical development will be stopped on the CNGA3 achromatopsia program as no clinical improvements were seen.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Applied Genetic Technologies is also developing AGTC-401 and AGTC-402 for the treatment of achromatopsia.

Review top news and interview highlights from the week ending February 4, 2022.

The investigational therapy has received orphan drug designation from the FDA for Stargardt disease as well as retinitis pigmentosa.
Kimmtrak is now the first approved therapy for unresectable or metastatic uveal melanoma.
The clinical trial of 4D-150 expects to enroll around 60 participants with wet AMD.

Both the Sirius and Celeste phase 2/3 trials are evaluating QR-421a.

Review top news and interview highlights from the week ending January 28, 2022.