
The OPTIC phase 1 study showed that the treatment was associated with a significant reduction in treatment burden after 1 injection.

The OPTIC phase 1 study showed that the treatment was associated with a significant reduction in treatment burden after 1 injection.

The FDA’s designation follows positive data presented at the 2020 ASH meeting.
The CEO of Avamab Pharma discussed how the field of gene therapy has shifted due to the COVID-19 pandemic.
The director of the Mario Lemieux Center for Blood Cancers at UPMC Hillman Cancer Center discussed the phase 2 CARTITUDE-2 trial.

The head of neuroscience at Sangamo Therapeutics discussed advances in understanding disease biology that allow for more specific gene targeting.
The phase 3 ZUMA-7 trial met both its primary and secondary endpoints.

The professor of medicine and director of the Hematopoietic Stem Cell Transplantation Program at University of Chicago Medicine described the impact of CAR T-cell therapy on the hematologic cancer landscape.
Patients receiving omidubicel also spent less time in hospital following transplant.

The head of neuroscience at Sangamo Therapeutics discussed the potential of ZFPs in central nervous system disorders.
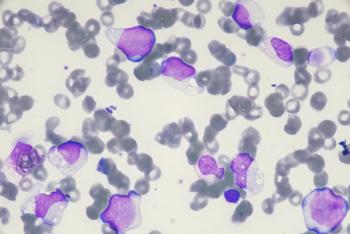
Conversion from MRD positivity to MRD-negative disease in a high-dose patient prompted the expansion of the trial population.
Surveyed oncologists supported the use of CAR T-cell therapy at higher rates than previous years.
A novel combination immunotherapy enables CD19-directed CAR T-cell therapies to target and eradicate difficult-to-treat solid tumors.

Bradley Monk, MD, FACOG, FACS, discusses the mechanism of VB-111 in patients with ovarian cancer.
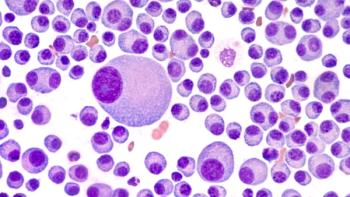
The recommendation comes after positive data was released from the recent phase 2 KarMMa trial.

Interim data from a phase 1 study from Intellia Therapeutics and Regeneron Pharmaceuticals is the first to support in vivo CRISPR genome editing in humans.

Philippe Moreau, MD, discussed the CARTITUDE-1 trial's promising results with ciltacabtagene autoleucel read out at the 2021 ASCO Annual Meeting.

The director of Clinical Research in Hematologic Malignancies at Levine Cancer Institute discussed adverse events in the CARTITUDE-1 trial.
The announcement follows positive results from the phase 1/2a trial of MCO-010 for RP.

The head of global research and executive vice president of Atara Biotherapeutics discussed the development of ATA188.

The chief executive officer of Gamida Cell discussed upcoming research on 2 of their investigational agents, GDA-201 and omidubicel, for hematological malignancies.
Corlieve Therapeutics’ lead program, AMT-260, previously demonstrated proof-of-concept in preclinical studies of temporal lobe epilepsy.
Peter Riedell, MD, discussed the need for more research in mantle cell lymphoma and access to novel treatments, including CAR T-cell therapies.

Flexion Therapeutics believes that local administration of a gene therapy may not only improve pain management but potentially slow disease progression.
uniQure announced positive results from their phase 3 HOPE-B study of etranacogene dezaparvovec.

Newly published data continues to demonstrate Zolgensma's efficacy in presymptomatic and symptomatic SMA Type 1.

The chief executive officer of Gamida Cell discussed the company's novel approach to cell proliferation.

Jasper Therapeutics and Aruvant Sciences are studying the use of JSP191 used with ARU-1801 in patients with sickle cell disease.

C. Ola Landgren, MD, PhD, discussed the role of CAR T-cell therapies in multiple myeloma.
James Hoffman, MD, discussed the significance of the approval of ide-cel for patients with multiple myeloma.
Faith E. Davies, MD, discussed the potential of CAR T-cell therapies for the treatment of multiple myeloma.