
An autologous lentiviral-based gene therapy demonstrated significant benefit, including high overall and event-free survival rates, immune reconstitution, and metabolic correction, for patients with ADA-SCID.

An autologous lentiviral-based gene therapy demonstrated significant benefit, including high overall and event-free survival rates, immune reconstitution, and metabolic correction, for patients with ADA-SCID.

Researchers observed 20-25% frequencies of this 4.9 kb deletion in HSPCs prior to xenotransplantation and at 17 weeks post-engraftment.

Nina Shah, MD, discusses the newfound role of BCMA-directed CAR T-cell therapies in multiple myeloma, how the field may select between ide-cel and cilta-cel if the latter is approved, and novel cellular therapies in clinical development.

Early results from the MOMENTUM study show that treated patients experienced fewer vaso-occlusive episodes and related hospital admissions.
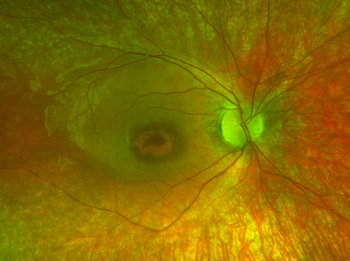
In a presentation at ARVO, Friederike Kortuem, MD, MSc, explains that treatment with voretigene neparvovec-rzyl led to a short-term change in the foveal morphology in a patient with visual impairment that included nyctalopia and decreased visual acuity in early childhood.
Strategies for improving outcomes across hematologic cancers and solid tumors range from addressing cytokine release syndrome and neurotoxicity mediators, immune rejection, on-target off-tumor toxicity, post-infusion control limitation, and immunosuppressive tumor microenvironment.

A podcast interview with study author Paul Yang, MD, PhD, on the current research and future implementation of the agent.

Arsen Osipov, MD, discusses the potential for ongoing research with immunotherapeutic approaches in gastrointestinal malignancies, including CAR T-cell therapy, bispecific T-cell engagers, and vaccines.

Based on findings from the SPEARHEAD-1 trial, Adaptimmune plans to submit a biologics license application to the FDA in 2022 for afamitresgene autoleucel to treat synovial sarcoma.

Investigators used an adeno-associated viral vector to deliver a normal functioning copy of the RPGR gene via subretinal injection.

Lineage Cell Therapeutics CEO Brian Culley shares a clinical trial update on their leading cell therapy candidate and discusses the important role of the FDA as more players enter the cell and gene therapy space.

The single-injection investigative gene therapy may help with providing continuous expression of aflibercept.

Dr. Abramson discusses the significance of the FDA approval of lisocabtagene maraleucel in refractory large B-cell lymphoma and provided insight into the efficacy and safety profiles of the CAR T-cell therapy as reported in the TRANSCEND NHL 001 trial.

The AAV8 vector–based gene therapy was associated with improvements in 2 measures of visual function.

A non-viral gene therapy sustained drug-delivery product that delivers anti-VEGF to the eye may replace the need for repeated intravitreal anti-VEGF injections and improve vision in patients with wet AMD.

Optical coherence tomography characteristics may be predictive of the efficacy of intravitreal injection of allogeneic human retinal progenitor cells, according to research presented at ARVO 2021.

The full regulatory approval of agalsidase beta has blocked the accelerated approval pathway for AVR-RD-01 as a treatment for patients with Fabry disease.

Approach can unravel causes in MYOC and TBK1 glaucoma.

A marketing authorization application has been submitted to the European Medicines Agency for the approval of the CAR T-cell therapy ciltacabtagene autoleucel in the treatment of patients with relapsed and/or refractory multiple myeloma.

Patients with the GBA mutation of Parkinson disease (PD) were shown to exhibit more severe cognitive decline than patients with idiopathic PD and those with both the GBA and LRRK2 G2019S mutations of PD.

Sequential therapy with CD19.28ζ-directed CAR T cells followed by allogeneic hematopoietic stem cell transplant induced durable disease control in a significant population of children and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia.

Remestemcel-L reduced mortality through 60 days in a prespecified group of ventilator-dependent patients below the age of 65 with COVID-19 and moderate or severe acute respiratory distress syndrome.

Apic Bio plans to initiate a phase 1/2 clinical trial in late 2021 or early 2022 as a multi-center, 3-part study to evaluate APB-102 in patients with SOD1-ALS mutations.

A patient enrolled in the phase 2 INFINITY trial exploring ADVM-022 for diabetic macular edema experienced a suspected unexpected serious adverse reaction, which has led to an unmasking of the study.

The FDA has granted an orphan drug designation to the cell therapy ITIL-168 as a potential treatment for patients with stage IIB to IV melanoma.

The CEO of Lineage Cell Therapeutics discusses their unique approach to addressing degenerative vision loss in dry age-related macular degeneration.

Ajeet Gajra, MD, MBBS, FACP, talks about identifying and removing the barriers for offering CAR T-cell therapy at the community practice level.

The FDA has lifted a clinical hold placed on an IND for VY-HTT01 in Huntington disease, allowing Voyager Therapeutics to initiate a phase 1/2 study for the agent later this year.

A policy allowing enforcement discretion for cellular-derived therapies, including stem cell-based products, will end on May 31, 2021, allowing the FDA to take more action against unapproved regenerative medicines.

The FDA has lifted a clinical hold on the development of etranacogene dezaparvovec for patients with hemophilia B.