A budget impact analysis suggest the burgeoning drug class may not be as available as it could be for sickle cell patients.

A budget impact analysis suggest the burgeoning drug class may not be as available as it could be for sickle cell patients.

The AAV-mediate gene therapy BS01 allowed 4 patients with retinitis pigmentosa who had complete or near-complete blindness to perceive light and motion.
Follow-up of 4.8 years to a phase 1 trial demonstrated that deep and durable responses are attainable in a large cohort of children and young adult patients with B-cell acute lymphoblastic leukemia who were treated with CD19-directed CAR T-cell therapy followed by allogeneic hematopoietic stem cell transplantation.

Multiple myeloma is currently incurable with many patients experiencing relapses.

Etranacogene dezaparvovec was "highly unlikely" to be the cause of hepatocellular carcinoma in a patient with hemophilia B enrolled in the phase 3 HOPE-B trial, according to an independent investigation.

The FDA has approved idecabtagene vicleucel as the first BCMA-directed CAR T-cell therapy for patients with relapsed/refractory multiple myeloma after 4 or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.

The tumor infiltrating lymphocyte therapy LN-145 was feasible, safe, and effective in patients with pretreated advanced cervical cancer.

The FDA has granted an orphan drug designation to the CD34+ cell therapy CLBS12 as a potential treatment for patients with thromboangiitis obliterans.

In 2021, Abeona Therapeutics is anticipating to complete enrollment in its phase 3 VIITAL study and additional findings for ABO-102 and ABO-101.

NurOwn was safe and led to improvements in function and cognition at 28 weeks compared with baseline for patients with progressive multiple sclerosis.

As a multi-systemic disorder, Fabry disease can impact the functions of various organs. As a genetic disorder, Fabry can impact the offsprings, parents, and relatives of the patient. Therefore, its effects are far-reaching.

RP-L301 demonstrated improvements in hemoglobin at 3- and 6-months for the first 2 patients treated in a phase 1 study for pyruvate kinase deficiency.

The FDA has granted a fast track designation to the placental-derived natural killer cell therapy CYNK-001 as a potential treatment for adult patients with recurrent glioblastoma multiforme.

Penn Medicine's Stephen Schuster, MD, offers an overview of an eventful year in CAR T-cell therapy at the NCCN Virtual Annual Meeting.

SGT-001 elicited promising improvements in functional and biomarker end points for patients with Duchenne muscular dystrophy.

SRP-9003 elicited sustained protein expression in muscle tissue and stabilized North Star Assessment for Dysferlinopathies scores at 2 years in patients with limb-girdle muscular dystrophy Type 2E.

Matthew Lunning, DO, discusses the findings from pivotal CAR T-cell therapy trials in LBCL, the potential to utilize these agents in the outpatient setting, how off-the-shelf allogeneic products could further transform outcomes, and the importance of shortening brain-to-vein time for this patient population.

Updated findings from across a number of studies highlighted the significant benefits of onasemnogene abeparvovec for children with spinal muscular atrophy.

Ninety percent of patients with cerebral adrenoleukodystrophy who were treated with elivaldogene autotemcel were alive and free of major functional disabilities at 2-years.

AB-205 showed an encouraging safety profile along with a robust ability to eliminate oral and gastrointestinal severe regimen-related toxicities in patients with systemic lymphoma who were undergoing high-dose therapy and autologous hematopoietic stem cell transplantation.

Omidubicel significantly improved median time to neutrophil engraftment compared with standard umbilical cord blood transplantation in patients with hematologic malignancies.
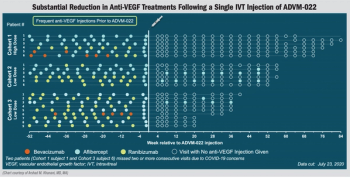
Investigators observe dramatic decrease in treatment burden seen in OPTIC study.
Axicabtagene ciloleucel elicited a high objective response rate of 85%, with a complete response rate of 74% when used as a first-line therapy in patients with high-risk large B-cell lymphoma.
A risk-adapted CD19 CAR T-cell therapy dosing approach allowed pediatric patients with relapsed/refractory acute lymphocytic leukemia to maintain a high rate of remission, while reducing the risk of severe toxicities among those with a high initial disease burden

The modified red blood cell therapy RTX-240 demonstrated promising efficacy and a favorable safety profile in patients with relapsed/refractory solid tumors.

The FDA has granted VX-880 a fast track designation as a potential treatment for patients with type 1 diabetes.
An experimental safety switch incorporated into chimeric antigen receptor (CAR) T-cell therapy reduced the severity of the treatment’s side effects in an early-phase trial.

Idecabtagene vicleucel induced progression-free survival up to 20 months and a complete response in one-third of patients with multiple myeloma who had relapsed multiple times and had received at least 3 previous lines of treatment.

The lentiviral vector used to create LentiGlobin was deemed a very unlikely cause of AML in a patient with sickle cell disease.

Christopher D'Angelo, MD, discusses emerging CAR T-cell therapies in the space, as well as in follicular lymphoma, and the challenges with using a 5-drug regimen.